Aviation And Travel Stocks Surge After Preliminary Results Show Pfizer Covid-19 Vaccine 90% Effective
- Pfizer announces that preliminary data shows their Covid-19 vaccine to be 90% effective.
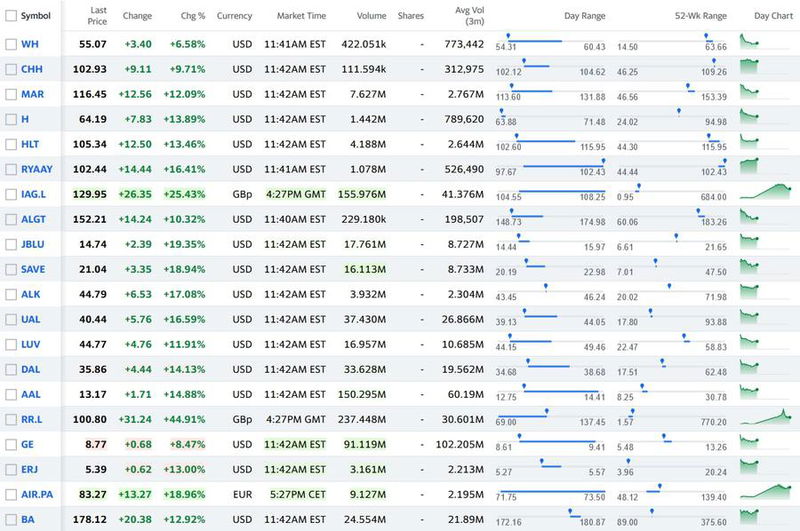
- Airline and aerospace stocks prices increased by at least 10% in response to the announcement.
- Pfizer expects to apply for emergency use authorization from the FDA by the third week of November.
- Subscribe to our daily newsletter for travel and aviation news and reviews.
After almost a year dealing with the Covid-19, several pharmaceutical and biotech companies have been racing to create a vaccine for the virus which has infected over 50 million people globally to date, and taking the lives of at least 1.2 million.
From a travel point of view, many of the associated sectors were forced to shrink by at least 50-80% of their capacity when compared to their performance in 2019. As governments continue trying to maintain a balancing act between sustaining their economies and keeping its citizens safe, travel globally still remains stifled due to border closures and general consumer fear of contracting the disease.
Close to 200 vaccines are currently in different stages of development, with 11 of those in Phase 3 (large-scale efficacy) trials.
Pfizer announces that preliminary data shows their Covid-19 vaccine to be 90% effective
Pfizer announced on Monday that preliminary results from their trials show their Covid-19 vaccine (being developed with partnership with German biotechnology firm BioNTech) to be 90% effective based on a sample group of over 44,000 people spread across the globe.
“Today is a great day for science and humanity. The first set of results from our Phase 3 COVID-19 vaccine trial provides the initial evidence of our vaccine’s ability to prevent COVID-19,” said Dr. Albert Bourla, Pfizer Chairman and CEO. “We are reaching this critical milestone in our vaccine development program at a time when the world needs it most with infection rates setting new records, hospitals nearing over-capacity and economies struggling to reopen. With today’s news, we are a significant step closer to providing people around the world with a much-needed breakthrough to help bring an end to this global health crisis. We look forward to sharing additional efficacy and safety data generated from thousands of participants in the coming weeks.”
From the 44,000+ taking part in the phase 3 trial, only 94 participants contracted Covid-19. Under 10% of those infected had received two shots of the vaccine, while the remaining 90% received placebo shots.
The news sent waves through the industry as most analysts believed that initial vaccines being pushed to the market might have a lower effectiveness rate of around 60-70%, while the Food and Drug Administration (FDA) predicted an even lower 50% effectiveness rate.
Airline and aerospace stocks surge in response to the announcement
On top of the results coming out of the US elections, a number of aviation and travel related companies saw 10%+ increases in their share prices in response to Pfizer’s announcement. The Dow Jones was up nearly 1,700 points (6%) while the S&P 500 climbed by 4%.
While stock prices provide no real indicator of how successful the industry will be in the next few months, the spike in share prices does show that this is the start of the news people have been waiting on for almost a year.
What’s next?
Monday’s announcement marks a big step for Pfizer, but the company said it will wait until the third week of November to submit an application for emergency use authorization from the FDA. They say that by then, the company would have two months of follow up data from at least half of its participants. They also added that the trial will continue, and that more people will be onboarded until they hit 164 infections.
There are still many questions to be answered, but Kathrin Jansen, head of vaccine research and development at Pfizer said that there have been no noted adverse reactions to the vaccine. Some candidates reported pain at the injection site, fevers, chills, headaches and fatigue, all of which lasted for about a day or two after receiving doses of the vaccine.
Even with expedited timelines in play, the pharmaceutical corporation will still have to wait for regulators to approve the vaccine before countries can start their distribution campaign. Assuming that all goes to plan, experts believe that only a limited supply will be made available before the end of the year.
Pfizer expects to product 50 million doses by the end of 2020, and an additional 1.3 billion in 2021.
It is expected that those most vulnerable to the disease will be given priority including those over the age of 50, as well as front line medical staff.
Pfizer is not the only firm developing a vaccine, but if the company’s vaccine is able to make it to the market by the end of the year, it could significantly shorten the recovery period for those in the travel industry. As it stands, many airline execs do not foresee a full recovery until 2024, but the progress announced in Monday’s news could potentially result in at least a year or two being shaved off those predictions.



